Benadryl Recall: Over 2,000 Bottles Pulled Due to Child Poisoning Risk The recall affects 100-milliliter bottles sold on Amazon from July 2023 to October 2024

Over 2,000 Benadryl Liquid Elixir bottles were recalled due to non-child-resistant packaging

Sujata Jana / Getty Images
- Over 2,000 Benadryl Liquid Elixir bottles were recalled due to non-child-resistant packaging.
- The recall affects 100-milliliter bottles sold on Amazon from July 2023 to October 2024.
- Consumers can get a refund by emailing Arsell Inc. with proof of disposal.
More than 2,000 bottles of Benadryl Liquid Elixir have been recalled over a potential poisoning risk to young children, the Consumer Product Safety Commission (CPSC) announced Thursday.
Benadryl Liquid Elixir contains diphenhydramine, an antihistamine, which must be in child-resistant packaging, according to requirements by the Poison Prevention Packaging Act. The packaging for the recalled 100-milliliter bottles was not child-resistant and posed the risk of child poisoning, the announcement said.
Arsell Inc., of Brooklyn, New York, initiated the recall, which affects certain bottles of Benadryl Liquid Elixir sold on Amazon.com.
No injuries or incidents have been reported in connection to the recalled medication.
Which Bottles of Benadryl Were Recalled?
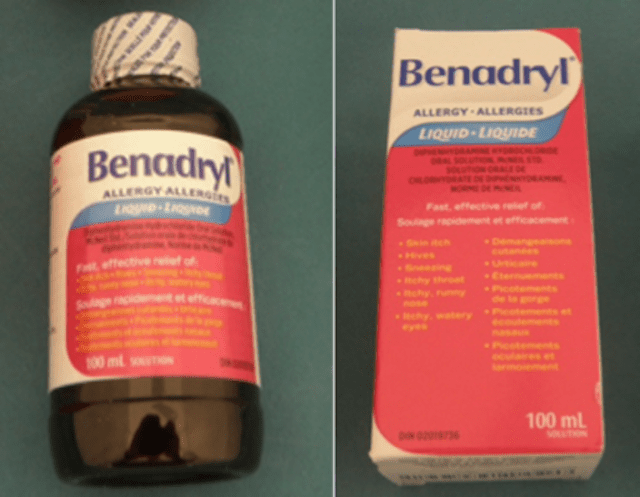
The recall affects Benadryl Liquid Elixir medication in 100 milliliter bottles sold in a paper box with the code "X003VRIGUL” on the label.
The bottles themselves are round and made of dark plastic. On the front, they have a pink and white label and the word "Benadryl" in blue lettering.
CPSC
According to the CPSC announcement, about 2,300 bottles of Benadryl Liquid Elixir are affected by the recall. The bottles were sold on Amazon.com from July 2023 through October 2024 for $16 to $19.
Why Was Benadryl Recalled?
The bottles of Benadryl Liquid Elixir were recalled over a packaging issue.
Benadryl Liquid Elixir contains the ingredient diphenhydramine, an antihistamine that's used in some allergy and sleep medicines. The ingredient can be harmful in large amounts, especially for children.
Because of this, the Poison Prevention Packaging Act requires that medication containing diphenhydramine be secured in child-resistant packaging.
The recalled bottles were not child-resistant, which posed a risk to children.
The CPSC recall announcement noted that only the bottles were recalled, not the medication itself, though both should be thrown away.
What to Do if You Have Benadryl at Home
According to the CPSC announcement, anyone who has the 100-milliliter bottles of Benadryl Liquid Elixir at home should immediately secure them out of reach from children and contact Arsell for a full refund.
Although only the bottles are affected by the recall, both the bottles and medication should be thrown away.
To receive the refund, consumers must submit their Amazon.com order number and a photo showing they disposed of the recalled Benadryl to [email protected].
Edited by Health with a background in health, science, and investigative reporting. Previously, she wrote full time about parenting issues for the app Parent Lab. Before that, she worked as a reporter for National Geographic covering wildlife crime and exploitation." tabindex="0" data-inline-tooltip="true"> Jani HallThis story originally appeared on: Health News - Author:Amber Brenza


















