How to fix a gut microbiome ravaged by antibiotics

A diet rich in diverse carbohydrates outperforms faecal transplants in mice at restoring microbial diversity, which has been linked to a range of health conditions
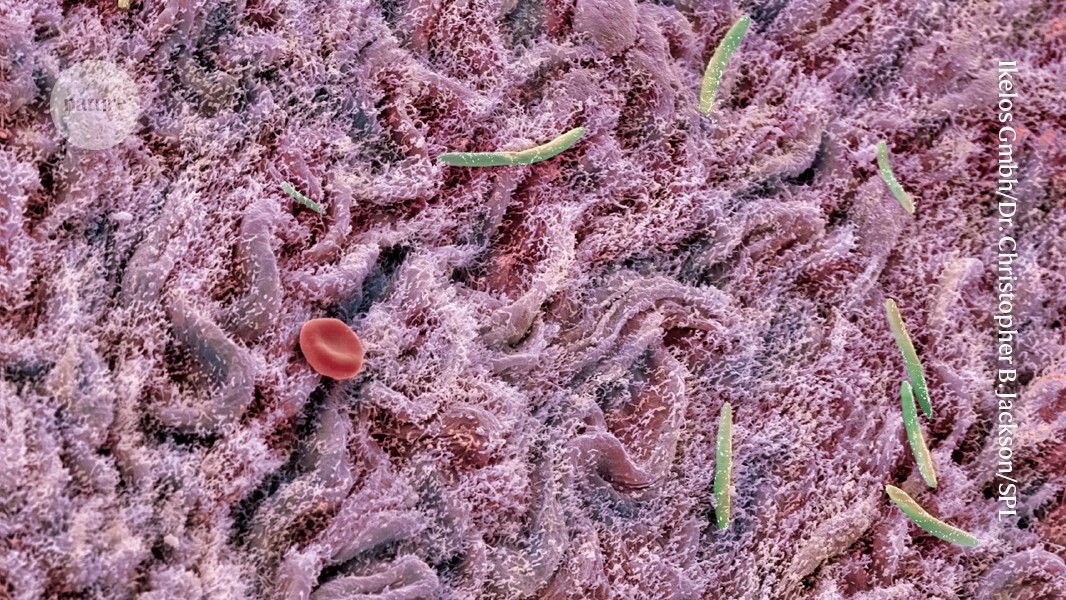
Bacteria (green; artificially coloured) populate a mouse’s intestinal lining. A healthy diet can restore gut microbes ravaged by antibiotics.Credit: Ikelos Gmbh/Dr. Christopher B. Jackson/Science Photo Library
A diet rich in fibre and low in fat can replenish populations of gut microbes ravaged by antibiotics, according to a study1 in mice.
The analysis, published on 30 April in Nature, found that good nutrition is more powerful than transplanting faeces — and the microbes it contains — from a healthy gut into a disrupted one. In addition, failure to correct an unhealthy diet rendered such transplants useless for helping the gut to recover from antibiotics.
The data thus far come only from mouse experiments but should inspire further work in humans, says Marie Joossens, who studies the gut microbiome at Ghent University in Belgium and was not involved in the study. “A faecal transplant sounds like a great shortcut,” she says. “But it’s a bit of wishful thinking to believe that it can be a quick fix. Nutrition plays such an important role.”
Biodiversity benefits
Microbes that reside in the gut influence digestion, immunity and the severity of a variety of health conditions, including Crohn’s disease and colon cancer. The key to fostering a healthy gut microbiome is cultivating diverse intestinal flora, says Megan Kennedy, a co-author of the paper who studies gut microbes at the University of Chicago in Illinois.
But in the United States and some other countries, people tend to consume a ‘Western’ diet that is high in simple, refined sugars, and low in the diverse forms of complex fibre found in fruits and vegetables. Eating a wide range of these fibres allows many species of gut bacterium to flourish, says Kennedy. “But when there’s only one type of resource, the species that’s best at eating it is going to be really good at that, and nothing else can compete.”
The result is a disrupted gut microbiome dominated by only a few species, a condition called dysbiosis. Antibiotics can also cause dysbiosis, as can some chronic diseases. That dysfunctional gut microbiome creates an environment that is easier for dangerous pathogens to colonize and infect, says study co-author Eugene Chang, a gastroenterologist also at the University of Chicago.
First principles
Researchers have tried to use faecal transplants to treat dysbiosis caused by chronic conditions, such as obesity, with little lasting success. Kennedy decided to study the recovery of the dysbiotic gut much as an ecologist might investigate the regrowth of a forest after a wildfire.
With Chang and other colleagues, she catalogued the microbes that disappeared when she switched them from healthy feed to a Western diet, or treated mice with antibiotics. The team then monitored the waves of microbes that returned after the researchers fed antibiotic-treated mice a healthier diet, gave them a faecal transplant or both.
Enjoying our latest content?
Login or create an account to continue
- Access the most recent journalism from Nature's award-winning team
- Explore the latest features & opinion covering groundbreaking research
or
Sign in or create an accountdoi: https://doi.org/10.1038/d41586-025-01313-7
This story originally appeared on: Nature - Author:Heidi Ledford


















