Sharp resolution, big samples: ExA-SPIM microscope accelerates brain imaging

An innovative microscopy technique bridges the gap between field of view and resolution
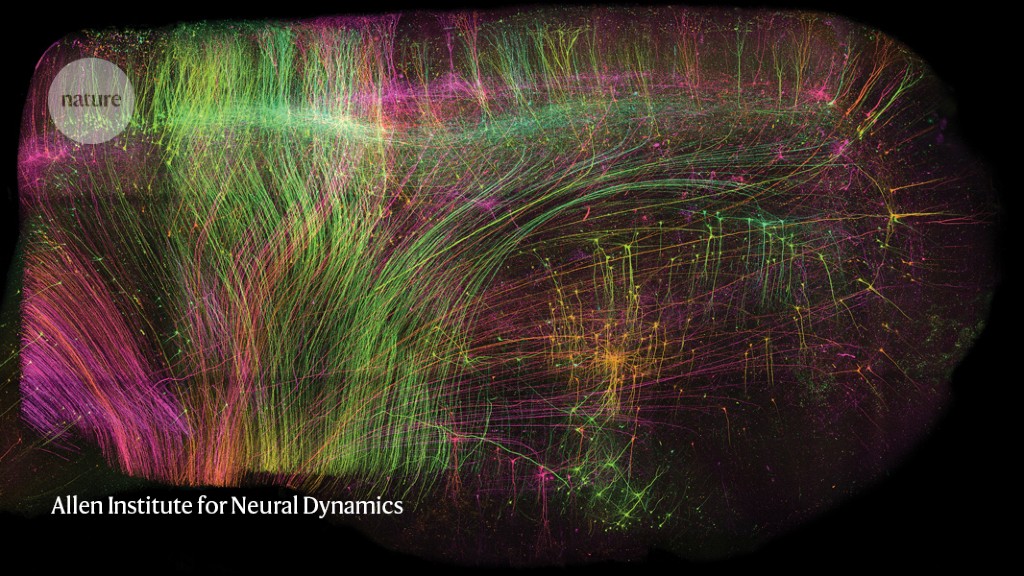
ExA-SPIM imaging of neurons in a piece of macaque brain measuring 1 x 1 x 1.5 centimetres.Credit: Allen Institute for Neural Dynamics
The mammalian brain is a multiscale system. Neuronal circuitry forms an information superhighway, with some projections potentially stretching dozens of centimetres inside the brain. But those projections are also just a few hundreds of nanometres thick — about one one-thousandth the width of a human hair.
Understanding how the brain encodes and transmits signals requires the alignment of events at both these scales. Conventionally, brain researchers have tackled this using a multi-step process: slice tissue into thin sections, image each section at high resolution, piece the layers back together and reconstruct the paths of individual neurons.
Zhuhao Wu, a neuroscientist at Weill Cornell Medicine in New York City, describes this last step as “like tracing a telephone wire in Manhattan”. In fact, he adds, it’s “even more complicated, since every neuron makes thousands, if not tens of thousands, of connections”.
Microscope makers have sought to allow researchers to take a wide-angled peek at a large chunk of tissue and still see the details up close, without having to first slice up the tissue and then reconstruct the axons across different sections. The challenge is that microscope objectives are generally designed in such a way that it is difficult to take high-resolution images of large samples.
A preprint published in June offers a solution1. First, the researchers chemically removed the lipids to make the tissue transparent. Then, they embedded it in a material called a hydrogel, which absorbs water, to expand the tissue to three times its original volume. Finally, they scanned it with a lens borrowed from a completely different field of science. In this way, it was possible to image whole mouse brains without the need for any slicing, and at a resolution of about 300 nm in the imaging plane and 800 nm axially (perpendicular to the plane), comparable to that of confocal microscopy, a technique widely used for high-resolution brain imaging. Called ExA-SPIM (expansion assisted selective plane illumination microscopy), the protocol was also used to image neurons in macaque and human brains.
“The big thing that such a system brings is the combination of being able to image very large volumes [of brain tissue] at very fine resolution,” says Jayaram Chandrashekar, a neuroscientist at the Allen Institute for Neural Dynamics in Seattle, Washington, who co-led the study with two colleagues: microscope developer Adam Glaser and institute head Karel Svoboda.
The approach can image an entire mouse brain in under a day — much faster and at higher resolution than is possible with other whole-brain approaches that have been applied to axon projections, such as MouseLight and fMost, which use tomography, says Chandrashekar. And the fact that images require only limited computational reconstruction significantly increases the accuracy of the resulting data.
Wu notes that none of the system’s components is new — they are just assembled in a synergetic way. “It’s not the first attempt to do this but it is probably the best attempt that we have now,” he says.
Step by step
The first element of the protocol — tissue clearing and expansion — has been used for decades, but usually on smaller pieces of tissue. The team had to optimize the technique to make sure that the brain samples expand isotropically — that is, by the same amount in all directions, says Glaser.
But the heart of the new method is the lens, Glaser says. In choosing it, they looked to the machine vision and metrology industries, settling on one that is normally used to identify pixel-sized defects in flat-panel displays and other electronic devices as they move along a conveyor belt. The lens has a larger field of view than do those typically used in the life sciences, he says, and because the tissue is expanded before imaging, the one-micrometre resolution is sufficient to trace an axon across the entire brain.
The researchers built that lens into a microscope that images 3D structures as a series of 2D sheets, a technique called selective plane illumination. They then added a camera — also taken from the machine vision and metrology industries — that has 38 times more pixels than do cameras conventionally used in the life sciences and can capture a field of view that measures 10.6 mm × 8.0 mm. With these specifications, a 2D sheet of an expanded mouse brain can be captured in about 15 tiles, compared with 400 tiles for a conventional microscope, Chandrashekar says.
“I liked that they thought outside the box and looked into other fields of science,” says Flavie Lavoie-Cardinal, a neuroscientist and microscopist at the University of Laval in Quebec City, Canada. “That is way better than approaches involving customized lenses,” she adds, which would make the system less accessible to other researchers.
Katrin Willig, a microscopist at the Georg-August University of Göttingen, Germany, says ExA-SPIM “is definitely of interest”, particularly for studies of brain connectivity, or ‘connectomics’. But, she adds, “it would be nice to improve the resolution a bit further to be able to clearly see dendritic spines.”
Data trove
According to Glaser, a single brain can be imaged in less than a day. The team has so far imaged 25 or so mouse brains, producing some 2.5 petabytes of data, which the team compresses five-fold and stores in the cloud. For data analysis, the researchers collaborate with Google, which provides machine-learning algorithms for processing the data and reconstructing images of the neurons.
The amount of data involved could represent a significant hurdle for would-be users, says Hari Shroff, a microscopist at Janelia Research Campus in Ashburn, Virginia. Apart from the challenge of analysing this volume of data, just moving it from the microscope to a computer is a big lift, he says. “Consortia of labs or institutes could invest in the people, the power or the infrastructure to do that, but it’s not trivial to handle,” he says.
That said, the microscope design is open-source and instructions for building it are available on GitHub. But Glaser says that the current version is a prototype that he plans to streamline and document over the next year. “It takes time to assemble, and it’s not super-easy to build,” he says. So far, several labs have expressed interest, but none has tried setting up the system. “We are going to redesign and re-engineer the microscope and document it extremely well — and hopefully even receive funding to disseminate the system so that other groups can adopt it.”
Tracing long-range neuronal connections is the most obvious application, and it’s the one the researchers are currently pursuing, says Chandrashekar. But they are also reworking the clearing and expansion protocols to keep biomolecules such as RNA and proteins intact, and allow them to be localized as well.
“I’m looking forward to seeing how other general neuroscience problems could also benefit from this technology,” says Wu.
doi: https://doi.org/10.1038/d41586-023-02570-0
This story originally appeared on: Nature - Author:Alla Katsnelson

















