What is an embryo? Scientists say definition needs to change

Lab-grown structures with the potential to develop into fetuses should be defined — and regulated — as embryos, some researchers say
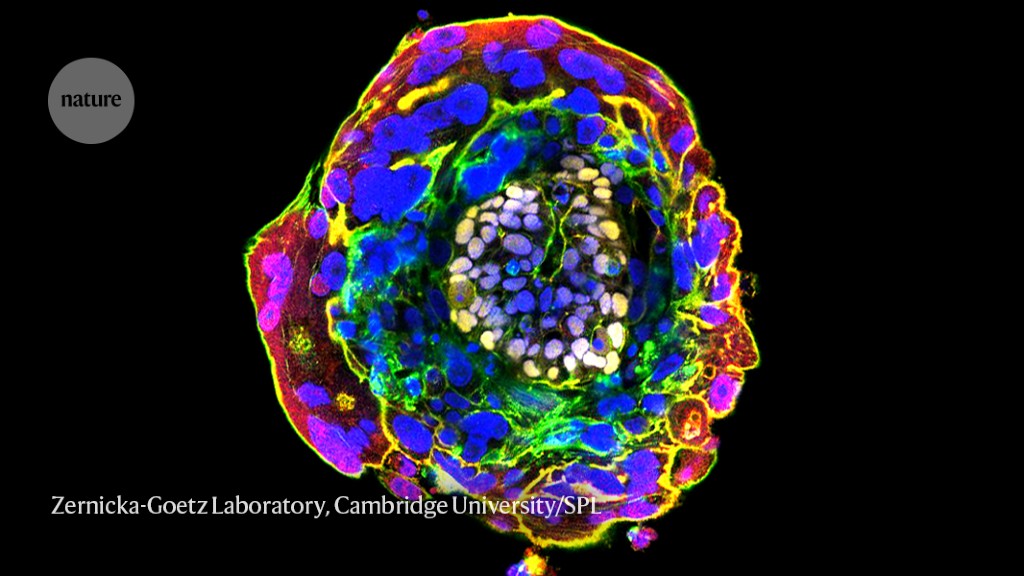
Eleven days after fertilization, the cells in this human embryo have begun to differentiate and self-organize.Credit: Zernicka-Goetz Laboratory, Cambridge University/Science Photo Library
It is time for a redefinition of the human embryo, a team of researchers has proposed. Advances in recent years have shown that human stem cells can be used to make embryo-like structures, called embryo models, that can recreate some features of early embryo development. Such research raises ethical dilemmas because these entities don’t meet formal definitions of embryos, so are not covered by regulations governing embryo research.
In a paper published in Cell on 17 August1, biologist Nicolas Rivron at the Institute of Molecular Biotechnology in Vienna and his colleagues suggest a new definition of human embryos that would include embryo models if they acquire the potential to develop into a fetus.
Stem-cell researcher Berna Sozen at Yale University in New Haven, Connecticut, says that a redefinition of the embryo would be timely, “not only to better reflect our current knowledge but also to pave the way for more accurate and inclusive discussions within the scientific community”.
Clusters of cells
Embryo models are clusters of embryonic stem cells (ESCs) that can begin to differentiate and organize themselves in ways that resemble the development of an early embryo. (They can also be made from induced pluripotent cells — mature cells reprogrammed into a stem-cell-like state.) Embryo models also include cells that are the progenitors of supporting tissues in the uterus, such as extra-embryonic cells that form a yolk sac and trophoblast stem cells that produce the placenta. These other cell types can also be made from ESCs.
Some researchers are using embryo models to study embryo development without the ethical and legal constraints that apply to real embryos. Currently, many countries follow the recommendation of the International Society for Stem Cell Research (ISSCR) that no human embryo may be cultured outside the body beyond 14 days after fertilization. The limit means that research on later developmental stages — which could help researchers to understand the causes of miscarriages and developmental defects — rely mainly on animal models, which are not always reliable guides to human development.
Last year, researchers in the United Kingdom and Israel reported mouse embryo models that could develop to a stage equivalent to an embryo 8.5 days after fertilization2,3 — approaching half of the gestation period. The embryo models had a body axis and nascent head, limbs and heart. Human embryo models have not yet got that far, but this year the same two groups reported human embryo models cultured in vitro to the equivalent stage, 13–14 days after fertilization4,5.
Such embryo models, resembling embryos after the stage at which they have implanted in the uterus, could not develop into fetuses even if they were to be implanted into a womb (a procedure that would be illegal for humans in many countries). But embryo models of the pre-implantation stage five to seven days post-fertilization, called blastoids, might be able to continue further along the developmental trajectory6. The ISSCR calls these integrated embryo models, and recommends that they be used in research only after careful review by scientific and ethical committees.
Tipping point
“It is now clear that scientific advances are narrowing the biological and therefore ethical and legal gaps between embryo models and embryos,” Rivron and his colleagues wrote in the Cell paper. “In the future, embryo models may pass a ‘tipping point’ after which, in our view, most of the ethical distinctions with an embryo would disappear.”
Last April, researchers showed that blastoids made from monkey ESCs and other cell types could induce pregnancies when implanted in monkeys, although the pregnancies all aborted spontaneously7. “We can foresee that the most complete embryo models will at some point tip over to become embryos giving rise to individuals,” says Rivron.
Legal definitions of embryos vary between countries, but are generally designed to refer to those made either by fertilization of an ovum by sperm, or by cloning — for example, by transferring a nucleus from a non-reproductive cell to an egg. No definition currently includes embryo models, says Alfonso Martinez Arias, a developmental biologist at the University Pompeu Fabra in Barcelona, Spain, and co-author of the Cell study.
Rivron, Martinez Arias and their colleagues argue that whether or not an embryo model could grow to at least the fetal stage should be the key issue for deciding on its moral and ethical status. They propose that an embryo be defined as “a group of human cells supported by elements fulfilling extraembryonic and uterine functions that, combined, have the potential to form a fetus ... regardless of how they came into being”. Precisely which fetal stage this refers to should be a topic for further discussion, they say.
Too soon?
Alison Murdoch, who researches reproductive medicine at Newcastle University, UK, says that the proposal “will be critical” in a planned review of embryo-research regulations by the Human Fertilisation and Embryology Authority, an independent UK regulator.
But Sozen stresses that “none of the current human embryo models even come close to meeting this threshold”. And Jacob Hanna, a stem-cell biologist at the Weizmann Institute of Science in Rehovot, Israel, says that it might be too soon for the field to start formalizing such distinctions. “Embryo models are at very rudimentary stage,” he says, “and trying to make changes at such an early stage can create unwanted or misleading outcomes that are hard to resolve later.”
“Currently, the formation of integrated embryo models requires the use of naive cells that rapidly accumulate genetic abnormalities and are too abnormal to form a fetus,” says Rivron. But he adds that “we need to think ahead to the possibility that these technical obstacles can be removed”.
Martinez Arias says that what counts as a genuine embryo model, rather than as simply a cell or tissue culture, also needs careful consideration. Otherwise, “we are going to confuse the scientists and the public”, he says.
doi: https://doi.org/10.1038/d41586-023-02641-2
This story originally appeared on: Nature - Author:Philip Ball


















