Superstar porous materials get salty thanks to computer simulations

Model predicts the structure of previously elusive compounds with practical applications
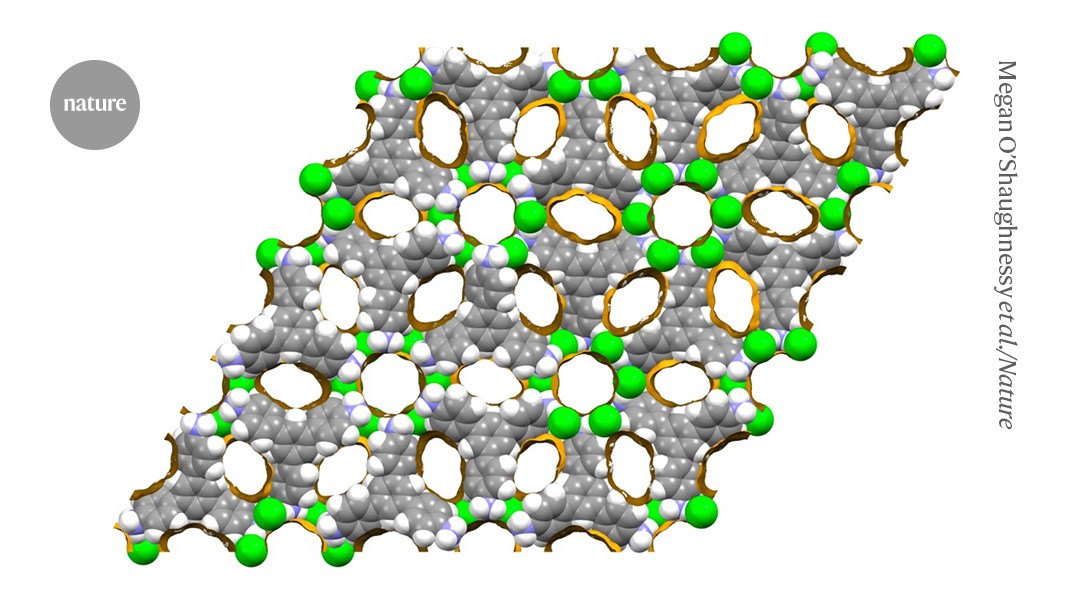
A computer simulation depicts a non-metal organic framework material that is comprised of chloride nodes (green) and amine linkers and is studded with large pores (yellow; carbon in gray, hydrogen in white, nitrogen in purple).Credit: Megan O’Shaughnessy et al./Nature
The revolutionary materials called metal–organic frameworks have taken chemistry by storm. Their highly porous structure makes them useful in venues from laboratory to factory, prompting scientists to create thousands of varieties. Now, a team has used computer simulations to design frameworks that skip the metal and are instead made entirely from organic salts1.
Salt-based frameworks could be cheaper to manufacture than their metal–organic counterparts and could have qualities that existing frameworks lack. The simulations have already yielded salt-based frameworks that can capture a pollutant produced in nuclear waste.
The research was published on 22 May in Nature.
Modular materials
Metal–organic frameworks, or MOFs, are built from cage-like modules. Each module is defined by metal ions or clusters that serve as nodes, linked by rigid carbon-based connectors — think of the hubs and rods in construction toys or scaffolding. The spaces between these components can hold other molecules, so MOFs can be used to filter and separate chemicals. Their modular construction has made it possible for scientists to create more than 90,000 types2 and has propelled the development of analogous frameworks made from other materials.
Frameworks made from salts, chemicals that are held together by ionic attraction between positively and negatively charged components, could have physical properties that are distinct from those of existing porous materials. Another benefit is that most organic salts are made of abundant elements, so there’s no need for the rare or expensive metals found in many MOFs.
But despite multiple attempts to make porous salt crystals over the past three decades, they remain few in number and much less stable than MOFs.
That’s partly because salt frameworks are intrinsically more difficult to design from scratch, says Graeme Day, a chemist at the University of Southampton, UK, and a co-author of the latest study. The ionic bonds between the charged particles that comprise salt crystals don’t follow the same geometric rules as the metal–organic bonds key to MOFs, and thus are much more variable. That means “it’s almost impossible to intuitively predict how the different components of these crystals are going to pack together”, Day says.
Simulating salts
To address this challenge, Day, chemist Andy Cooper at the University of Liverpool, UK, and their colleagues turned to a computational technique called crystal structure prediction. They applied this to work out how to make frameworks from salts called ammonium halides, in which the positively charged linkers are nitrogen-containing amine molecules and the negatively charged nodes are halide ions such as chloride or bromide.
The authors adapted the technique to simulate the complex interactions between halide nodes and various types of ammonium linker. This allowed the team to screen possible frameworks virtually to determine the best candidates to try making in the lab.
Guided by these calculations, the team settled on linkers called TT, TTBT and TAPT, each of which has three arms ending in a nitrogen group. The simulations predicted that these linkers would form stable frameworks with defined pores that varied in volume depending on the size of the linker.
Simple synthesis
The researchers made the compounds by mixing up solutions of the chosen linker and adding either hydrochloric acid or hydrobromic acid, drop by drop, at room temperature. “Once we got the prediction, the synthesis was really very simple,” Cooper says. Using a suite of high-resolution measurements, the team confirmed that the crystal structures of the synthesized materials matched those of highly stable porous arrangements predicted by the simulations. The researchers call the materials non-metal organic frameworks.
To explore the applications of non-metal organic frameworks, the researchers tested their ability to capture iodine — a highly desirable property, because nuclear waste often contains radioactive iodine. They found that all three materials performed just as well as, if not better than, most MOFs.
Tom Woo, a computational chemist at the University of Ottawa, says the iodine-uptake experiments are a compelling demonstration of how this type of material could become very useful, especially given how easy it is to synthesize.
K. Travis Holman, a chemist and materials scientist at Georgetown University in Washington DC, says the findings illustrate a combination of computational prediction and synthesis that has “unparalleled” power. Both Woo and Holman agree that these methods create a new avenue for the rational design of porous salt frameworks and will accelerate the development of materials.
doi: https://doi.org/10.1038/d41586-024-01481-y
Read the related News & Views ‘Designer porous solids open up vast sandbox for materials research’
This story originally appeared on: Nature - Author:Ariana Remmel


















